DESCRIPTION
This product is a biomaterial for tissue restoration with a derivative of cross-linked sodium hyaluronate originated from bacterial fermentation. It is injected into the skin layer of facial wrinkles and is used for temporal wrinkle improvement.
COMPOSITION
WARNINGS AND INCOMPATIBILITIES
- Be sure to understand the user's manual before use.
- Inspect the sealed inner packaging before use for damage or defects; do not use if any are found.
- Check the expiration date on the product before use.
- Because it is a disposable sterile product, it must not be reused and do not re-sterilize.
- Do not bend or break the needle for use.
- Contraindicated for patients with: hypersensitivity to hyaluronic acid, history of severe allergy or anaphylaxis, tendency for keloid/hypertrophic scar, bleeding-related disorder, bacterial allergy.
- If injected into blood vessels, serious side effects (e.g., blindness) may occur. Avoid use in thin-skin areas with high vascular injection risk; use special caution.
- Do not use near blood vessels (periocular site) to avoid vision impairment and tissue necrosis.
- Should not be used in combination with laser treatment, chemical peeling, dermabrasion, or other surgical methods.
- Excessive use should be avoided; do not inject to epithelium.
CAUTIONS
- Procedure should be performed by a doctor sufficiently trained in treatment using this product.
- Before the procedure, inform the patient about indications, contraindications, and potential side effects.
- Confirm no product damage; check expiration date.
- Observe patient’s skin condition after the procedure.
- Resolve issues such as pimples or open pores before the procedure to prevent leakage.
- Disinfect injection site and surrounding tissue before use.
- If injected to the epithelium, blanching occurs; inject into the dermis.
- When combined with other anesthetics, pay attention to systemic toxicity; high doses may cause CNS or cardiovascular toxicity.
- Medication may cause local redness or hypersensitivity.
- Before the procedure, prohibit aspirin, NSAIDs, or vitamins.
- Recommend no makeup for 24 hours post-procedure; avoid sunlight, extreme cold, and sauna for 2 weeks.
INTERACTIONS
Hyaluronic acid may cause precipitation with quaternary ammonium compounds such as benzalkonium chloride (sanitizer/disinfectant). Do not use in combination with, or allow contact between, these substances (or instruments that contacted them) and this product.
SIDE EFFECTS
The practitioner should explain that the following symptoms may appear immediately after injection or later and to seek care if symptoms persist.
- Bruise, inflammation, edema (swelling), pain, skin discoloration.
- Allergic reactions, inflammatory reactions (abscess), infections, tissue necrosis at injection site.
- Filler leakage through an opened procedure site; movement to another location; permanent bump formation.
- Visual impairments, including blindness, particularly with intravascular injection or periocular use.
In case inflammation or other side effects persist for more than one week, the patient should consult a doctor at once and receive treatment.
Adverse events reported with systemic lidocaine
- Shock, malignant hyperthermia (rare), CNS symptoms (drowsiness, dizziness, anxiety, apnea, convulsions, coma), cardiovascular symptoms (hypotension, bradycardia, cardiac arrest), hypersensitivity (rash, hives, edema, anaphylactic shock).
METHOD OF USE
- Use by licensed medical professionals only. Thoroughly read the user manual about indications, contraindications, and potential side effects.
- Check product integrity and expiration date before use.
- Inject into the dermis; disinfect thoroughly prior to injection.
- Connect the enclosed needle and remove air; replace if contaminated.
- Inject an appropriate amount as required; massage lightly after injection to shape the area.
STORAGE AND DISPOSAL AFTER USE
- Store sealed at 2°C–25°C; avoid direct sunlight and freezing.
- Do not reuse; discard used syringes, needles, and remaining gel after treatment according to local regulations.
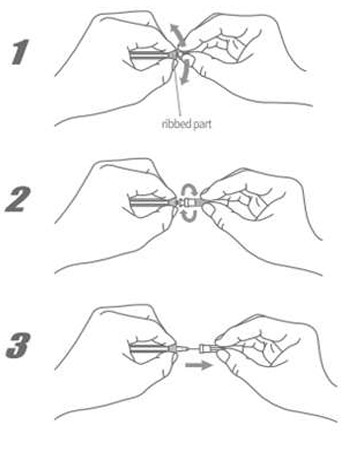
ASSEMBLY OF NEEDLE TO SYRINGE
For safe use, properly assemble the needle to the syringe.
- Hold the luer lock adapter of the syringe between the index finger and thumb.
- With the other hand, firmly push and screw the needle on clockwise. Improper assembly can cause needle disengagement or leakage.
- Remove the protective cap by holding the luer-lock and pulling in opposite directions.
DIAGRAM
SYMBOLS USED ON LABELING