INTENDED USE
The products are designed to temporarily improve facial wrinkles in adults.
INGREDIENTS AND CONTENTS
Lidocaine hydrochloride 3mg/mL
Hyaluronic acid 10mg/mL
Lidocaine hydrochloride 3mg/mL
Hyaluronic acid 10mg/mL
Lidocaine hydrochloride 3mg/mL
Glutathione 0.1mg/mL
Niacinamide 10mg/mL
I. INSTRUCTIONS FOR USE
A. Preparations Before Use
- Check the expiration date and inspect the product for any damage. Do not use if the product is expired, damaged, or contaminated.
- Physicians should carefully read and understand the Instructions for Use enclosed with this product, and use the product only as instructed. The manufacturer shall not be held responsible for any issues arising from use not in accordance with the Instructions for Use.
B. Storage and Handling Instructions After Use
- This product is a sterile, single-use medical device and must be discarded immediately after use.
- Do not re-sterilize and reuse.
II. PRECAUTION
A. Contraindications
- The product is contraindicated for the following patients:
- Patients with a history of anaphylaxis or severe systemic allergic reactions.
- Patients who are known to be hypersensitive to hyaluronic acid, polynucleotide sodium or mannitol. (Only applicable to models containing the corresponding ingredient.)
- Patients with a history of autoimmune diseases or a compromised immune system.
- Patients with liver dysfunction or blood coagulation disorders.
- Patients with acute rheumatic fever due to cardiac complications.
- Patients with a history of keloid formation, hyperpigmentation, or hypertrophic scars, or who are prone to developing them.
- Patients with a history of Streptococcus diseases.
- Pregnant or breastfeeding women.
- Minors (as defined by local regulations)
- Patients with an allergic reaction to the reagents and solutions used in the manufacturing process.
- Patients with permanent skin-expanding implants, such as hardened gel or silicone, inserted in the facial area.
- Patients with a history of side effects from EMLA cream.
- Patients with clinically significant disorders in the cardiovascular, gastrointestinal, endocrine, or central nervous systems.
- The products must not be injected into blood vessels.
- Do not inject excessive amounts.
- The product must not be administered to areas other than those intended for its intended use.
- The product must not be administered to areas affected by skin diseases until infection and inflammation have been resolved.
- Do not use this product in conjunction with treatments such as laser therapy, deep chemical peels, or dermabrasion.
B. Warning
- Do not use if the product is damaged.
- Check the expiration date on the product label.
- Do not re-sterilize.
- Do not reuse.
- Do not use in combination with or mix with other products.
- Discard the syringe and remaining product after use.
C. Precaution for Use
- This product must be administered by a physician who has received adequate training in the injection technique.
- Prior to the procedure, the physician must adequately inform the patient about the indications, contraindications, and potential side effects of the product.
- Before use, check that the sterility has not been compromised.
- Check the expiration date of the product.
- Do not inject into blood vessels where there is a risk of vascular obstruction and resulting tissue necrosis.
- Intravascular injection may result in serious side effects, including blindness. Therefore, it is recommended to avoid use in areas with thin skin and high likelihood of vascular injection, such as the glabellar, periorbital region, and lips. Special caution is required during treatment.
- Do not use until infection and inflammation have been controlled or resolved.
- The safety and efficacy for lip augmentation have not been established.
- Herpetic eruptions may recur following injection in patients with a history of herpetic eruptions.
- Safety has not been established in patients who are prone to keloid formation, hyperpigmentation, hypertrophic scars.
- The safety and effectiveness of long-term use have not been established.
- The safety of multiple injections of this product has not been established.
- This product must be injected intradermally only.
- Anatomical knowledge of the treatment area is required to avoid perforation or compression of blood vessels, nerves, or other vulnerable structures. Special caution must be exercised during the procedure.
- Patients with a history of streptococcal diseases (e.g., recurrent sore throat, acute rheumatic fever) should undergo a skin hypersensitivity test (dual test) before the treatment.
- Patients who are taking anticoagulant medications or substances that may prolong bleeding should be informed that the risk of hematoma and bleeding may increase during the procedure.
- Patients should avoid taking NSAIDs (e.g., aspirin) and excessive amounts of vitamins prior to the procedure.
- It is recommended that patients avoid applying makeup for 24 hours after the procedure and refrain from prolonged exposure to sunlight, UV rays, extreme cold, or saunas for 2 weeks.
- It is crucial to ensure proper attachment of the needle and syringe, as improper connection may lead to separation during the treatment.
- If the needle is clogged, do not apply excessive pressure and replace the needle immediately.
- Athletes should be aware that they may test positive in a doping test due to this product.
- Be careful not to contaminate the treatment area when using this product.
- Use this product in a safe and appropriate environment, such as an aseptic area or treatment room.
- As no studies have been conducted on potential interactions with other implants, this product should not be injected into areas where other implants are present.
D. Incompatibility
- Sodium hyaluronate and polynucleotide sodium are incompatible with quaternary ammonium compounds such as the disinfectant Benzalkonium chloride. Ensure that instruments or products that have come into contact with this substance do not come into contact with this product.
E. Side Effect
- Before the procedure, the physician must fully explain the potential side effects to the patient. Side effects include, but are not limited to:
- After the procedure, symptoms such as itching, tenderness, bruising, edema, erythema, laxity, redness, and inflammation reactions (granulomas, nodules at the treatment site, infection, etc.) may occur and can last from a few hours to a week.
- Bleeding, hematoma, and allergic reactions at the treatment site.
- Hardening, discoloration, and insufficient effect at the treatment site.
- Necrosis of the glabellar area, granulomas, hypersensitivity, abscess, etc., has been reported with the use of other hyaluronic acid products.
- Movement to other areas from the treatment site.
- Visual impairment, including blindness, may occur when used around the eyes or nose.
- Disruption of blood supply caused by injections into blood vessels.
- Permanent lump formation at the treatment site.
- The patient should inform the physician if they experience inflammation or any other side effects that persist for more than one week, and the physician must treat them appropriately.
- Any other side effects associated with the use of this product should be reported to the distributor and manufacturer.
STORAGE
- Store at 2℃ to 25℃
- Fragile, handle with care
- Do not freeze, heat, or expose to light
LOT No. AND EXPIRATION DATE
Displayed at the bottom of package
WEIGHT AND PACKING UNIT
MANUFACTURED BY
Homecast Co., Ltd.
14F, Doosan Bldg, 726, Eonju-ro, Gangnam-gu, Seoul, Republic of Korea, 04792
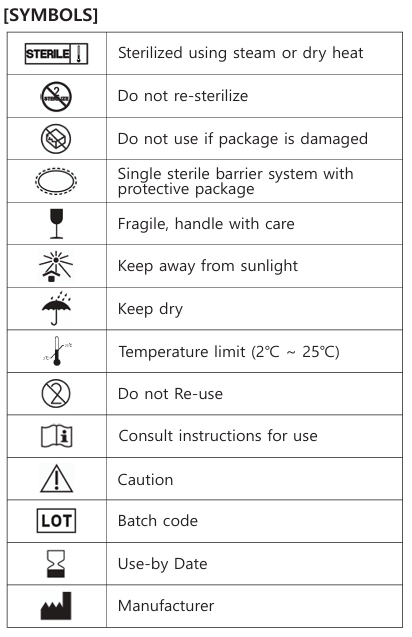
SYMBOLS